Palynology & Paleontology Lab
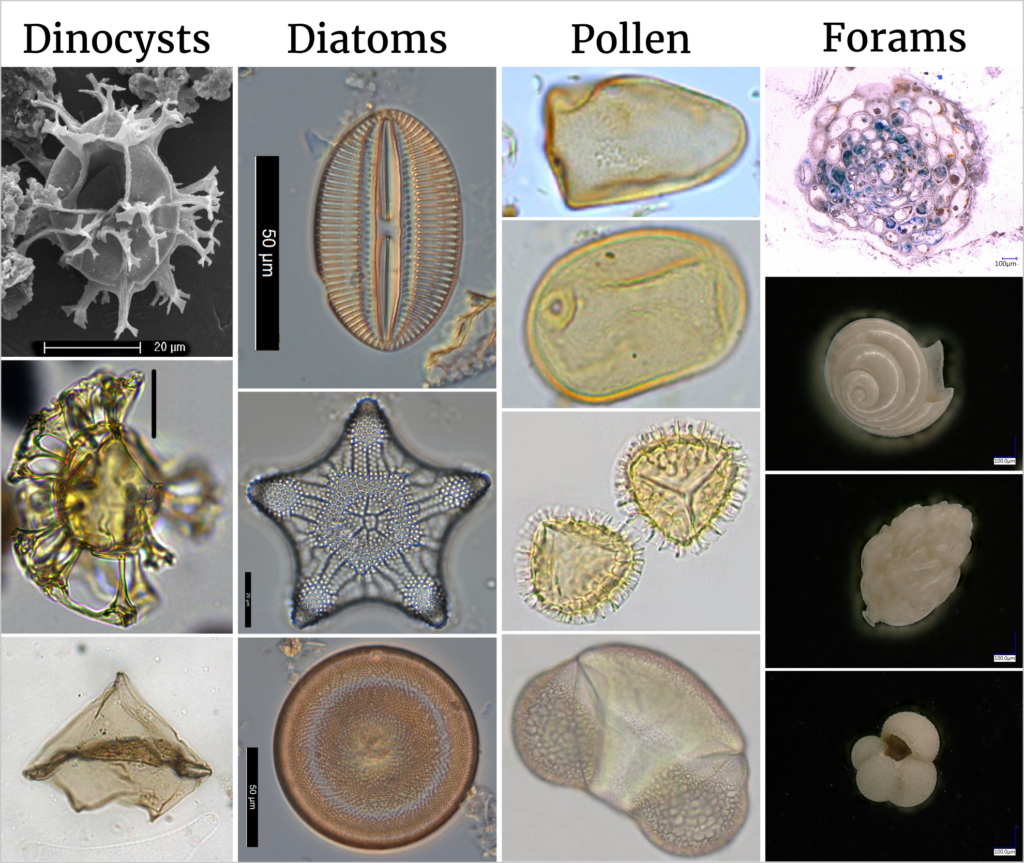
The GeoLab hosts one of the largest and most distinguished palynological laboratories in the world. The lab does not only offer the processing and preparation of recent and ancient pollen samples, but also of other fossil plant materials like leaves, needles and microorganisms like dinoflagellates, foraminifera and mussel crustaceans (Ostracoda). These methods help us to reconstruct past ecosystems and to study climate change. Dinoflagellate cysts (or dinocysts) are typically 15-100 µm in diameter and produced by planktonic organisms that have existed mainly in marine but also in fresh water environments from about 210 million years ago (late Triassic) up to the present day. From top to bottom: Spiniferites spp., Sangiorgia pospelovae (both Sluijs & Brinkhuis, 2024), Lejeunecysta (©Francesca Sangiorgi). Diatoms are microalgae generally 20-200 µm in size and they are surrounded by a cell wall made of silica, called a frustule. For 150-200 million years (early Jurassic), diatoms have been producing oxygen through carbon fixation. From top to bottom: Diploneis suborbicularis, Triceratium pentacrinus, Actinocyclus gallicus (©Kees Nooren). Pollen and spores directly relate to the reproductive stage in the life cycle of land plants and they offer a tool for direct land-sea correlation. From top to bottom: Cyperaceae, Secale cereale (Rye), Lycopodium (ground pines), Picea (Spruce) (©Timme Donders). Most Foraminifera (or forams) are marine organisms which produce shells commonly made out of calcium carbonate and they are usually less than 1 mm in size with a few exceptions. The majority lives on or within the seafloor (i.e., benthic), while a smaller number float in the water column (i.e., planktonic). From top to bottom: Planorbulinella caneae, Pteropod shell, Uvigerina peregrina, Globigerina bulloides (©Lucas Lourens). We offer several sample preparation methods to concentrate, clean and/or isolate microfossils on the 5th floor of the GeoLab. Depending on material type and process requirements. Concentration is done by removing other sedimentary components, either through dissolution with acids, separation with heavy liquids or by sieving and washing the samples. The remaining microfossils can then be inspected and enumerated with brightfield or scanning electron microscopes. Techniques that we use in our lab include: The lab has facilities to decalcify samples with hydrochloric acid prior to the analysis of organic C and/or N isotopes and to destruct sample material with HF/HClO4/HNO3 or Aqua Regia in preparation of elemental analysis. There are more methods available and we can assist in developing new customized methods. The Palynology and Paleontology Lab is the first lab of the Geolab that received a silver certificate according to the ‘Laboratory Efficiency Assessment Framework’ (LEAF) for its sustainable operations.
Technical experts:
Scientific advisor:
Contact:
Natasja Welters
Giovanni DammersFrancesca Sangiori
PalyFGStratP.Geolab@uu.nl

 Most of the minerals on Earth belong to the group of silicates. The silicates in our samples are removed by dissolution with a certain acid: hydrofluoric acid (HF). The corrosive nature of this acid that also attacks glass requires special facilities, protective measures and training. Our HF Lab is in a separate room on the 5th floor with purpose-built fume hoods with fume scrubbers that neutralize the fume exhausts. It is equipped for both warm and cold applications with HF. For safe working, there is sensitive gas detection, specific safety clothing, personal emergency sensors and an HF medical first aid kit within reach. Everyone working with HF will receive detailed instructions and training before their work. In this way, we keep our lab users safe while working with hazardous chemicals.
Most of the minerals on Earth belong to the group of silicates. The silicates in our samples are removed by dissolution with a certain acid: hydrofluoric acid (HF). The corrosive nature of this acid that also attacks glass requires special facilities, protective measures and training. Our HF Lab is in a separate room on the 5th floor with purpose-built fume hoods with fume scrubbers that neutralize the fume exhausts. It is equipped for both warm and cold applications with HF. For safe working, there is sensitive gas detection, specific safety clothing, personal emergency sensors and an HF medical first aid kit within reach. Everyone working with HF will receive detailed instructions and training before their work. In this way, we keep our lab users safe while working with hazardous chemicals.